Abstract
Introduction: First line therapy of AML has not changed significantly since the 1970s and still relies on cytarabine as its backbone. Older patients or patients with comorbidities have low tolerability to intensive cytarabine treatment due to increased morbidity and mortality related to its toxicity, such as cerebellar toxicity, bone marrow suppression, gastrointestinal toxicity and infections. Hence, currently available intensive cytarabine treatment is unjustified in this population due to its poor risk/benefit ratio, and therefore, older and unfit AML patients are usually treated with reduced intensity therapy with poor outcomes.
BST-236 is a new compound of cytarabine covalently bound to asparagine. It acts as a pro-drug of cytarabine, enabling delivery of high cytarabine doses to leukemia cells with lower systemic exposure to the free drug and relative sparing of normal tissues. As such, BST-236 may serve as an ideal therapy for leukemia, particularly for delivering high doses of cytarabine to medically unfit or older adults. The aim of this Phase I/II study was to evaluate the safety and optimal dose of BST-236 in refractory/relapsed or newly-diagnosed acute leukemia patients unfit for standard induction therapy.
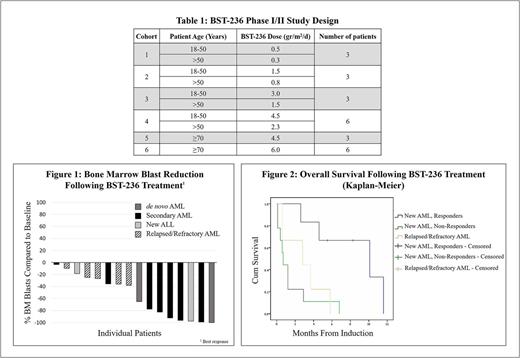
Methods: Acute leukemia patients, either relapsed/refractory, or older or unfit newly-diagnosed, were enrolled to a prospective open label single arm study. The study included 6 dose-escalating cohorts of patients treated with 6 daily doses of BST-236 administered as a 1-hour intravenous infusion. Cohorts 1-4 enrolled patients age ≥18 years, dosed with 0.5 g/m2, 1.5 g/m2, 3 g/m2 or 4.5 g/m2, respectively, with a 50% dose reduction in each cohort for patients age >50 years. Cohorts 5 and 6 enrolled patients ≥70 years of age, dosed with 4.5 g/m2 or 6 g/m2, respectively (Table 1).
Results: Twenty-six (26) patients received at least 2 daily doses of BST-236, twenty-three (23) of them (14 males and 9 females) completed at least one 6-day treatment course, including 2 patients who received 2 courses. Out of the 23 patients who completed at least one treatment course, median age 77 (range 27-90), 6 had relapsed/refractory AML following standard chemotherapy, median age 64 years (range 27-81), 15 were newly-diagnosed AML patients unfit for standard chemotherapy, median age 78 years (range 70-89), including 11 patients (73%) with AML secondary to myelodysplastic disorder (MDS) or myeloproliferative neoplasms, median age 77 years (range 70-89), and 2 patients with de novo ALL, age 79 and 90.
BST-236 treatment was well-tolerated and MTD was not reached in this study. Notably, the maximal dose of 6 g/m2/d BST-236 is the molar equivalent of 4 g/m2/d of cytarabine. Moreover, adverse events were mainly hematological "on-target" events, and no neurological events or >grade 2 events such as mucositis, diarrhea, or alopecia were reported in any of the cohorts during treatment or within 30 days of follow up.
Assessment of the outcome of cohort 6 is still ongoing and will be available in the coming weeks. The majority of the newly diagnosed AML patients in cohorts 1-5 responded to BST-236 (Figure 1). The overall response and Complete Remission (CR) rates, of the newly-diagnosed AML patients (de novo and secondary to MDS) was 71% and 43%, respectively. Follow up of the Overall Survival (OS) of these patients is still ongoing, and is currently 6.9 months (active) for all responders, 9.2 months (active) for CR patients, and 0.6 months for the non-responders (Figure 2). Notably, 67% of the responding patients had secondary AML, refractory to hypomethylating agents (HMA). No CR was reached in the 6 patients suffering from relapse or refractory AML, and their median OS was 2.3 months (Figure 2).
Conclusions: This Phase I/II study demonstrates that BST-236, a cytarabine pro-drug, is able to safely deliver high-dose cytarabine to older and unfit patients (median age 78) with no significant typical cytarabine toxicities other than "on-target" hematological events. Moreover, the general wellbeing of the patients during and after administration, as noted by the treating physicians, was exceptionally improved compared to intensive therapy. The good safety profile, accompanied by the high response rates and significantly prolonged survival of the older and unfit newly-diagnosed population, most of them refractory to HMA, is highly encouraging. A phase II study is planned to confirm these results.
Zuckerman: BioSight Ltd.: Consultancy. Gengrinovitch: Biosight: Employment. Flaishon: BioSight: Employment. Ben Yakar: Biosight: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal